ABSTRACT
Objective.
To describe the prevalence of leptospirosis in the Americas.
Methods.
A systematic review and meta-analysis, in the period 1930 to 2017, performed on a search of six platforms: PubMed, Web of Science, Scopus, Lilacs, Embase, and Cochrane.
Results.
The search found 77 publications of which 53 (68%) were from the period 2000–2017. Of the 77, 62 studies were included in the analysis, from North America (11, 17%), Central America (9, 14%), and South America (42, 67%), and 22 studies were from urban areas. Leptospirosis prevalence in the 62 studies analyzed corresponded to 28% (95% CI [23, 32]). Countries with higher prevalence were United States of America (41%), Colombia (29%), and Brazil (21%). The most frequent serovars found were Icterohaemorrhagiae (43 of 77 publications, 55%), Canicola (35, 45%), Pomona (28, 36%), and Grippotyphosa (26, 33%).
Conclusions.
There is variability of Leptospira species and serovars with heterogenous distribution throughout the Americas, with high prevalence in some countries, highlighting the need for action to control the disease.
Keywords
Leptospirosis; serogroup; prevalence; meta-analysis; Americas
RESUMEN
Objetivo.
Describir la prevalencia de la leptospirosis en las Américas.
Métodos.
Revisión sistemática y metanálisis correspondientes al período 1930-2017, mediante una búsqueda en seis plataformas: PubMed, Web of Science, Scopus, Lilacs, Embase y Cochrane.
Resultados.
En la búsqueda se encontraron 77 publicaciones, de las que 53 (68%) eran del periodo 2000-2017. En el análisis se incluyeron 62 de los 77 estudios, correspondientes a América del Norte (11, 17%), Centroamérica (9, 14%) y América del Sur (42, 67%), y 22 estudios correspondientes a zonas urbanas. La prevalencia de la leptospirosis en los 62 estudios analizados fue del 28% (IC del 95% [23, 32]). Los países con mayor prevalencia fueron Estados Unidos de América (41%), Colombia (29%) y Brasil (21%). Las serovariedades más frecuentes fueron icterohaemorrhagiae (43 de 77 publicaciones, 55%), canicola (35, 45%), pomona (28, 36%) y grippotyphosa (26, 33%).
Conclusiones.
Se observa variabilidad de especies y serovariedades de Leptospira, con una distribución heterogénea en las Américas y una elevada prevalencia en algunos países, lo que pone de manifiesto la necesidad de adoptar medidas para controlar la enfermedad.
Palabras clave
Leptospirosis; serogrupo; prevalencia; metaanálisis; Américas
RESUMO
Objetivo.
Descrever a prevalência da leptospirose nas Américas.
Métodos.
Uma revisão sistemática e metanálise referente ao período de 1930 a 2017, realizada por meio de busca em seis plataformas: PubMed, Web of Science, Scopus, Lilacs, Embase e Cochrane.
Resultados.
A pesquisa encontrou 77 publicações, das quais 53 (68%) eram do período de 2000 a 2017. Dos 77 estudos, 62 foram incluídos na análise, da América do Norte (11, equivalente a 17%), América Central (9, equivalente a 14%) e América do Sul (42, equivalente a 67%), e 22 estudos foram realizados em áreas urbanas. A prevalência da leptospirose nos 62 estudos analisados correspondeu a 28% (IC 95% [23, 32]). Os países com maior prevalência foram os Estados Unidos da América (41%), a Colômbia (29%) e o Brasil (21%). Os sorovares mais frequentes encontrados foram Icterohaemorrhagiae (43 de 77 publicações, equivalente a 55%), Canicola (35, equivalente a 45%), Pomona (28, equivalente a 36%) e Grippotyphosa (26, equivalente a 33%).
Conclusões.
Há variabilidade nas espécies e sorovares de Leptospira, que têm distribuição heterogênea nas Américas e alta prevalência em alguns países, o que destaca a necessidade de ações para controlar a doença.
Palavras-chave
Leptospirose; sorogrupo; prevalência; metanálise; América
Leptospirosis is a zoonotic disease present in all continents except Antarctica, caused by spirochete bacteria of the genus Leptospira. Leptospires have a diversity of animal reservoirs, with the brown rat, Rattus norvegicus, as a main reservoir (11. Brito T de. Pathology and pathogenesis of human leptospirosis. Rev Inst Med Trop Sao Paulo. 2018;60(April):e23err–e23err., 22. Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010 Jan;140(3–4):287–296.). The environment has a central role in infections of both animals and humans, as these occur mainly due to contact with contaminated environment, especially water. Clinical manifestations vary from subclinical to severe pulmonary hemorrhage (Weil’s disease), with lethality ranging from 5% to 40% (33. Gouveia EL, Metcalfe J, Carvalho ALF de, Aires TSF, Villasboas-Bisneto JC, Queirroz A, et al. Leptospirosis-associated Severe Pulmonary Hemorrhagic Syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14(3):505–508. https://doi.org/10.3201/eid1403.071064.
https://doi.org/10.3201/eid1403.071064... –55. Petersen AM, Boye K, Blom J, Schlichting P, Krogfelt KA. First isolation of Leptospira fainei serovar Hurstbridge from two human patients with Weil’s syndrome. J Med Microbiol. 2001;50(1):96–100.). The global average incidence of leptospirosis is around 1.9 cases per 100 000 population, and the prevalence varies according to each region, reaching between 11% and 30%. Other studies indicate a variation in incidence between 0.10 and 975 annual cases per 100 000 population (66. Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9(9):e0003898., 77. Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, et al. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl Trop Dis. 2015;9(10):e0004122. https://doi.org/10.1371/journal.pntd.0004122.
https://doi.org/10.1371/journal.pntd.000... ).
Some environmental factors such as climate, temperature, soil properties, humidity, and sanitary conditions can favor the prolonged persistence of Leptospira in the environment and transmission of leptospirosis (22. Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010 Jan;140(3–4):287–296., 88. Lehmann JS, Matthias MA, Vinetz JM, Fouts DE. Leptospiral pathogenomics. Pathogens. 2014;3(2):280–308. https://doi.org/10.3390/pathogens3020280.
https://doi.org/10.3390/pathogens3020280... , 99. Gomes MJP. Gênero Leptospira spp. Universidade Federal do Rio Grande do Sul: 2015. Available from: https://edisciplinas.usp.br/pluginfile.php/1605090/mod_folder/content/0/G%C3%AAnero%20Leptospira%20%202015.pdf.
https://edisciplinas.usp.br/pluginfile.p... ). Thus, it can be expected that tropical regions with high precipitation and inadequate sanitation systems present higher leptospirosis prevalence than areas without these conditions (1010. Desvars A, Cardinale E, Michault A. Animal leptospirosis in small tropical areas. Epidemiol Infect. 2011;139(02):167–188. https://doi.org/10.1017/S0950268810002074.
https://doi.org/10.1017/S095026881000207... , 1111. Martins G, Penna B, Lilenbaum W. Maintenance of Leptospira infection in cattle under tropical conditions. Vet Rec. 2010;167. https://doi.org/10.1136/vr.c5695.
https://doi.org/10.1136/vr.c5695... ).
In the transmission cycle of leptospirosis, humans are considered incidental hosts, given that they are not a definitive reservoir such as rats and other mammals, and infection commonly happens during recreational, occupational, or domestic activities (1212. Haake DA, Levett PN. Leptospirosis in Humans. Curr Top Microbiol Immunol. 2015;387:65–97. https://doi.org/10.1007%2F978-3-662-45059-8_5.
https://doi.org/10.1007%2F978-3-662-4505... , 1313. Ullmann LS, Langoni H. Interactions between environment, wild animals and human leptospirosis. J Venom Anim Toxins Incl Trop Dis. 2011;17(2):119–129.). The severity of the symptoms depends on factors such as epidemiological conditions, susceptibility, and bacterial virulence. Proximity with potential animal reservoirs such as cattle, dogs, and pigs increases the risk of Leptospira transmission and might favor an increase in the prevalence of the disease. The highest risk of complications from the disease is concentrated in individuals between 40 and 49 years of age, more frequently males, and complications are more severe in individuals older than 60 years (1212. Haake DA, Levett PN. Leptospirosis in Humans. Curr Top Microbiol Immunol. 2015;387:65–97. https://doi.org/10.1007%2F978-3-662-45059-8_5.
https://doi.org/10.1007%2F978-3-662-4505... ).
The serovars of greatest epidemiological importance for human leptospirosis, being responsible for the more severe outcomes, are Icterohaemorrhagiae, Canicola, and Grippotyphosa (1313. Ullmann LS, Langoni H. Interactions between environment, wild animals and human leptospirosis. J Venom Anim Toxins Incl Trop Dis. 2011;17(2):119–129., 1414. Levett PN. Systematics of Leptospiraceae. Curr Top Microbiol Immunol. 2015;387:11–20. https://doi.org/10.1007/978-3-662-45059-8_2.
https://doi.org/10.1007/978-3-662-45059-... –1616. Guernier V, Goarant C, Benschop J, Lau CL. A systematic review of human and animal leptospirosis in the Pacific Islands reveals pathogen and reservoir diversity. PLoS Negl Trop Dis. 2018;12(5):e0006503.). These serovars are the most frequent in humans. Among animals, there is specificity between serovars and host species. Serovars Pomona and Tarassovi are more frequent in pigs; Bratislava in horses; and Icterohaemorrhagiae, Australis, and Pomona in dogs. However, the mechanisms involved in this complex and specific process are unknown (1717. Cilia G, Bertelloni F, Fratini F. Leptospira Infections in Domestic and Wild Animals. Pathogens. 2020;9(7):573.). This study aims to describe the prevalence of leptospirosis in the Americas. Its results can help inform control measures that are more individualized and focused on local realities.
MATERIALS AND METHODS
This study is a systematic review and meta-analysis, performed following PRISMA guidelines. The protocol designed for the study is deposited in the PROSPERO database under protocol number CRD42020180359.
Databases
Data utilized in this study were extracted from research articles found in the platforms PubMed, Web of Science, Scopus, Lilacs, Embase, and Cochrane, from studies published between 1930 and 2017, in English, Portuguese, and Spanish. The search was performed using the descriptors: “Leptospir”; “Leptospira”; “Leptospiral”; “Leptospirosis”; among others. Whenever possible, we used the Medical Subject Headings method in searches, associated to an array of combinations of the Boolean operators “AND” and “OR.” In addition, the references lists of the articles and reviews on the theme in question were evaluated to identify studies not indexed by the databases but that could be relevant for inclusion in the review. The publications selected were managed using Mendeley, with removal of duplicates and application of inclusion criteria.
Eligibility criteria
The inclusion criteria adopted were: publications in English, Portuguese, and Spanish, with observational study designs (cohort, cross-sectional, or case-control). Studies with no defined etiological agent, in vitro assays, experimental studies, editorials, review articles, case reports, and studies from outside the Americas were excluded.
Selection of studies and data extraction
All publications identified in the databases were selected by reading the title and abstract in a process performed independently by two reviewers, with calculation of the kappa statistic for concordance after reading the title and abstract (kappa = 0.69). Publications that fulfilled the inclusion criteria were read in full. From the eligible studies, the author, year of publication, period, locality, study design, sampling, Leptospira serovars, and prevalence were obtained.
Risk of bias
The studies selected for the meta-analysis were evaluated for quality based on a scale prepared for prevalence studies described by Munn et al. (1818. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014 Aug;3(3):123–128.). The instrument evaluates the publications on nine criteria, including: adequation of sample size, validity of the method, description of the subjects, statistical analysis, and others. Scores are attributed as “yes,” “no,” “unclear,” and “not applied,” with more adequate studies receiving higher rates of “yes” responses. Risk of bias was considered high when the study had one or more “no” responses, and moderate when one or more outcomes were declared as “partial” or “unclear.” Low risk of bias was defined as most/all outcomes receiving a “yes” response.
Statistical analysis
The outcome for this study was the prevalence of leptospirosis in adults, with a 95% confidence interval (CI). We used the DerSimonian and Laird method to estimate the variability between studies, and heterogeneity was evaluated by Cochran’s Q test with magnitude defined by the I-square (I2) test. The prevalence estimated in the studies was obtained using the meta-analytical method of random effects for proportions, given the high heterogeneity in the estimations of each individual study.
The data from the studies included in the meta-analysis were logit-transformed to meet the requirement of normality of the meta-analytical model with random effects. Confidence intervals for the results of individual studies were calculated using the Clopper–Pearson method.
We performed two meta-regressions to identify the causes of heterogeneity, using the Knapp and Hartung tests to test the variables: sample size, region of the Americas, type of study, and risk of bias score. Publication bias was not evaluated, as it is not adequate for meta-analyses of prevalence (1919. Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014 Aug;67(8):897–903.).
All analyses considered p < 0.05 as statistically significant. All analyses were performed on STATA 12 (Stata Corp, College Station, TX, United States of America).
RESULTS
Characteristics of the studies
The database search resulted in 77 publications, with all being included in the meta-analysis, considering the inclusion criteria. Of these, 53 (68%) were published in the period 2000 to 2017, and the most frequent study design was cross-sectional, in 67 (87%) studies. However, 15 publications were excluded after the meta-regression for not presenting relevant data, leaving 62 articles for analysis (Figure 1) (see Table A1 in supplementary material).
Of the 62 articles included, studies were from North America (11, 17%), Central America (9, 14%), and South America (42, 67%). Studies were more frequently conducted in urban areas (22 studies) (Tables 1 and 2). The sample sizes included in the studies varied from 9 to 6 066 persons.
The serovars most frequently found were Icterohaemorrhagiae (in 43 of 77 [55%] publications), Canicola (35, 45%), Pomona (28, 36%), and Grippotyphosa (26, 33%) (Table 3).
Risk of bias
Twenty-four percent of the 77 publications found presented high risk of bias in all evaluation criteria, while 62% were within the expected quality criteria. The criteria with larger proportion of negative scores were: description of the characteristics of the study population (22%), description of the population sampling methods (21%), adequate sample size (15%), and adequate response rate (12%).
Prevalence
Average general prevalence for leptospirosis in the 62 studies analyzed was 28% (95% CI [23, 32]) (Figure 2). There was high heterogeneity in the prevalence reported in the studies, ranging from 1% to 98% (Figure 2). Analyzing the geographical distribution of leptospirosis prevalence in the Americas, we calculated that the prevalence was 28% in North America, 31% in Central America, and 26% in South America (Table 1). Colombia and Brazil were the countries with higher frequency of studies (17 and 16, respectively), with a prevalence of 29% and 21%, respectively (Table 2). Also, studies presented high leptospirosis prevalence in both urban and rural environments (Table 1).
Characteristics of the publications regarding regions of the Americas and study environment, 1930–2017
Thirty-eight serovars of Leptospira were found in the 77 publications examined. The serovars with highest prevalence were Icterohaemorrhagiae, Mankarso, Patoc, and Copenhageni (Table 3), while other serovars presented prevalence under 4%.
DISCUSSION
Leptospirosis was first described in rural environments, but with globalization the disease has become frequent in urban areas, especially in less-developed and developing nations with areas of low socioeconomic status and issues of poor sanitation (2020. Pereira MM, Andrade J. Epidemiological aspects of leptospirosis in a slum area in the city of Rio de Janeiro, Brazil. Search for leptospires and specific antibodies in rodents. Trans R Soc Trop Med Hyg. 1988 Sep;82(5):768–770. https://doi.org/10.1016/0035-9203(88)90231-3.
https://doi.org/10.1016/0035-9203(88)902... –2222. Santos NDJ, Sousa E, Reis MG, Ko AI, Costa F. Rat infestation associated with environmental deficiencies in an urban slum community with high risk of leptospirosis transmission Cad Saude Publica. 2017;33(2):e00132115.). In this context, cities that have households with little infrastructure (without road pavement and running water) and open sewer canals have endemic levels of leptospirosis. Such conditions provide harbor and food for rats, main reservoirs of Leptospira, favoring their reproduction and, consequently, increasing leptospirosis transmission to humans (2323. Hamond C, Martins G, Lawson-Ferreira R, Medeiros MA, Lilenbaum W. The role of horses in the transmission of leptospirosis in an urban tropical area. Epidemiol Infect. 2013;141(1):33–35.).
Some studies indicate that increasing population density, as well as constant rural exodus into urban centers, are factors that positively affect the risk of leptospirosis infection (2424. Lelu M, Muñoz-Zanzi C, Higgins B, Galloway R. Seroepidemiology of leptospirosis in dogs from rural and slum communities of Los Rios Region, Chile. BMC Vet Res. 2015;11:31.–2626. Lall C, Kumar KV, Raj RV, Vedhagiri K, Vijayachari P. Prevalence and Diversity of Leptospires in Different Ecological Niches of Urban and Rural Areas of South Andaman Island. Microbes Environ. 2016;31(1):79–82. https://doi.org/10.1264/jsme2.ME15149.
https://doi.org/10.1264/jsme2.ME15149... ). Hence, it is possible that disorganized population growth (the presence of large population conglomerates in a given region) and increase in the amount of inadequate housing can contribute to population growth of Leptospira reservoirs, thus increasing risk of leptospirosis transmission.
All studies retrieved, related both to urban and rural environments, report high prevalence of leptospirosis in domestic animals, making them important reservoirs in the leptospirosis epidemiological cycle and in the incidental transmission to humans (2727. Escandón-Vargas K, Osorio L, Astudillo-Hernández M. Seroprevalence and factors associated with Leptospira infection in an urban district of Cali, Colombia. Cad Saude Publica. 2017;33(5):e00039216.–3232. Barcellos C, Lammerhirt CB, Almeida MAB de, Santos E dos. Distribuiçäo espacial da leptospirose no Rio Grande do Sul, Brasil: recuperando a ecologia dos estudos ecológicos TT. Cad Saude Publica. 2003;19(5):1283–1292. https://doi.org/10.1590/S0102-311X2003000500007.
https://doi.org/10.1590/S0102-311X200300... ). Also, evidence indicates higher incidence of leptospirosis in tropical and subtropical regions. Our study corroborates these findings, as we find higher prevalence of leptospirosis in countries like Colombia and Brazil (29% and 21%, respectively), both having areas of hot and humid climate. In Brazil, agribusiness is an important factor to be considered regarding prevalence of leptospirosis, as domestic animals such as cattle, equines, and goats, and their proximity with humans, can increase disease transmission.
In Colombia, studies indicate human prevalence ranging from 6% to 35% (3333. Carreño LA, Salas D, Beltrán KB. Prevalencia de Leptospirosis en Colombia: revisión sistemática de literatura. Rev Salud Publica. 2017;19(2):204–209., 3434. Góngora A, Parra JL, Aponte LH, Gómez LA. Seroprevalencia de Leptospira spp. en Grupos de Población de Villavicencio, Colombia. Rev Salud Publica. 2008;10(2):269–278.). In Brazil, human prevalence varied between 10% and 19% in parts of the country (3535. Nicolino RR, Lopes LB, Rodrigues RO, Teixeira JFB, Haddad JPA. Prevalence and spatial analysis of antileptospiral agglutinins in dairy cattle - Microregion of Sete Lagoas, Minas Gerais, 2009/2010. Arq Bras Med Vet Zootec. 2014;66(3):648–654., 3636. Marteli AN, Genro LV, Diament D, Guasselli LA. Análise espacial da leptospirose no Brasil. Saude Debate. 2020;44(126):805–817.). This variation results from the heterogeneity of environmental characteristics, given that some regions of the same country may have variations, such as higher food offer and humidity, which can cause higher presence of reservoirs. Climate and soil characteristics might allow for longer survival of leptospires in the environment, increasing the chance of incidental transmission to humans (22. Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010 Jan;140(3–4):287–296., 1616. Guernier V, Goarant C, Benschop J, Lau CL. A systematic review of human and animal leptospirosis in the Pacific Islands reveals pathogen and reservoir diversity. PLoS Negl Trop Dis. 2018;12(5):e0006503.).
Icterohaemorrhagiae was the serovar of greatest clinical and epidemiological importance for humans; severe forms of the infection are reported, with negative prognosis and mortality around 50%. Despite Icterohaemorrhagiae being the most frequent serovar in the Americas (10% prevalence), countries in Africa and Oceania present higher prevalence for Pomona, Canicola, and Hardjo (3737. Allan KJ, Biggs HM, Halliday JEB, Kazwala RR, Maro VP, Cleaveland S, et al. Epidemiology of Leptospirosis in Africa: A Systematic Review of a Neglected Zoonosis and a Paradigm for ‘One Health’ in Africa. PLoS Negl Trop Dis. 2015;9(9):e0003899. Available from: https://doi.org/10.1371/journal.pntd.0003899.
https://doi.org/10.1371/journal.pntd.000... –3939. Machang’u RS, Mgode GF, Assenga J, Mhamphi G, Weetjens B, Cox C, et al. Serological and molecular characterization of leptospira serovar Kenya from captive African giant pouched rats (Cricetomys gambianus) from Morogoro Tanzania. FEMS Immunol Med Microbiol. 2004;41(2):117–121.).
Distribution of prevalence by Leptospira serovar in the publications analyzed (N = 77), 1930–2017
Some serovars are more frequent in animals than in humans, such as Patoc and Bratislava, both found in pigs and cattle. In spite of this preference, we highlight that humans can be infected by these serovars, even if accidentally, but the Patoc serovar is not epidemiologically relevant to the human health context. This reflects the importance of spatial overlap between humans, pathogens, and reservoirs in disease dynamics.
Limitations
This study has limitations that can influence the prevalence results, such as information bias and lack of prevalence data in some publications, which can underestimate the calculation of the meta-analysis. Also, some of the studies used fix serovar testing through a panel of 24 of the more frequent serovars, which can also influence the diversity of leptospiral serovars found in the literature. It is noteworthy that the studies had the same diagnostic criterion, the microscopic agglutination test (MAT), but despite this, the prevalence may vary depending on the selected population in each study.
Conclusion
Leptospirosis is distributed in North America, Central America, and South America; however, there is a higher number of studies from South America (in particular Colombia, with 29% prevalence of leptospirosis, and Brazil, 21%).
Icterohaemorrhagiae, Canicola, and Pomona were the most common serovars in the studies, corroborating recent findings. These are the main serovars associated with severe leptospirosis in humans.
In the Americas, leptospirosis prevalence is around 28%, which is concerning for epidemiologists and highlights the neglected status of the disease in several countries. Also, the circulation of pathogenic serovars highlights the precarious state of prevention and control measures in these territories. Leptospirosis is a neglected disease worldwide, with repercussions in human health, as well as being an economic burden in endemic countries.
Furthermore, although leptospirosis is a frequent disease in the population, its impacts are still superficially explored. Thus, it is important to understand that transmission is a dynamic phenomenon (human–animal–environment), and that more specific control actions might be necessary, such as health education campaigns and implementation of protocols to manage animals in confined/controlled spaces. Improving basic sanitation infrastructure is also needed, as leptospirosis is a disease linked to environmental and socioeconomic conditions. Thus, more studies should be carried out in this sense, to give greater visibility to this disease, which, despite being neglected, is important in public health.
Disclaimer.
The authors hold sole responsibility for the views expressed in the manuscript, which may not necessarily reflect the opinion or policy of the RPSP/PAJPH and/or those of the Pan American Health Organization (PAHO).
- Author contributions.All authors conceived the original idea. AB, ESB, and MP collected the data and analyzed the data. AB and ESB reviewed the titles and abstracts of the articles. CGZ, DO, ESB, and FC wrote the paper. All authors reviewed and approved the final version.
- Conflict of interest.None declared.
- Financial support.This work was supported by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB).
REFERENCES
- 1.Brito T de. Pathology and pathogenesis of human leptospirosis. Rev Inst Med Trop Sao Paulo. 2018;60(April):e23err–e23err.
- 2.Adler B, de la Pena Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010 Jan;140(3–4):287–296.
- 3.Gouveia EL, Metcalfe J, Carvalho ALF de, Aires TSF, Villasboas-Bisneto JC, Queirroz A, et al Leptospirosis-associated Severe Pulmonary Hemorrhagic Syndrome, Salvador, Brazil. Emerg Infect Dis. 2008;14(3):505–508. https://doi.org/10.3201/eid1403.071064
» https://doi.org/10.3201/eid1403.071064 - 4.dos Santos VM, dos Santos JA, Sugai TA, dos Santos LA. Weil's syndrome. Rev Cubana Med Trop. 2003;55(1):44–46. PMID: 15849953.
- 5.Petersen AM, Boye K, Blom J, Schlichting P, Krogfelt KA. First isolation of Leptospira fainei serovar Hurstbridge from two human patients with Weil’s syndrome. J Med Microbiol. 2001;50(1):96–100.
- 6.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015;9(9):e0003898.
- 7.Torgerson PR, Hagan JE, Costa F, Calcagno J, Kane M, Martinez-Silveira MS, et al. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl Trop Dis. 2015;9(10):e0004122. https://doi.org/10.1371/journal.pntd.0004122
» https://doi.org/10.1371/journal.pntd.0004122 - 8.Lehmann JS, Matthias MA, Vinetz JM, Fouts DE. Leptospiral pathogenomics. Pathogens. 2014;3(2):280–308. https://doi.org/10.3390/pathogens3020280
» https://doi.org/10.3390/pathogens3020280 - 9.Gomes MJP. Gênero Leptospira spp. Universidade Federal do Rio Grande do Sul: 2015. Available from: https://edisciplinas.usp.br/pluginfile.php/1605090/mod_folder/content/0/G%C3%AAnero%20Leptospira%20%202015.pdf
» https://edisciplinas.usp.br/pluginfile.php/1605090/mod_folder/content/0/G%C3%AAnero%20Leptospira%20%202015.pdf - 10.Desvars A, Cardinale E, Michault A. Animal leptospirosis in small tropical areas. Epidemiol Infect. 2011;139(02):167–188. https://doi.org/10.1017/S0950268810002074
» https://doi.org/10.1017/S0950268810002074 - 11.Martins G, Penna B, Lilenbaum W. Maintenance of Leptospira infection in cattle under tropical conditions. Vet Rec. 2010;167. https://doi.org/10.1136/vr.c5695
» https://doi.org/10.1136/vr.c5695 - 12.Haake DA, Levett PN. Leptospirosis in Humans. Curr Top Microbiol Immunol. 2015;387:65–97. https://doi.org/10.1007%2F978-3-662-45059-8_5
» https://doi.org/10.1007%2F978-3-662-45059-8_5 - 13.Ullmann LS, Langoni H. Interactions between environment, wild animals and human leptospirosis. J Venom Anim Toxins Incl Trop Dis. 2011;17(2):119–129.
- 14.Levett PN. Systematics of Leptospiraceae. Curr Top Microbiol Immunol. 2015;387:11–20. https://doi.org/10.1007/978-3-662-45059-8_2
» https://doi.org/10.1007/978-3-662-45059-8_2 - 15.Levett PN. Leptospirosis. Clin Microbiol Rev. 2001;14(2):296–326.
- 16.Guernier V, Goarant C, Benschop J, Lau CL. A systematic review of human and animal leptospirosis in the Pacific Islands reveals pathogen and reservoir diversity. PLoS Negl Trop Dis. 2018;12(5):e0006503.
- 17.Cilia G, Bertelloni F, Fratini F. Leptospira Infections in Domestic and Wild Animals. Pathogens. 2020;9(7):573.
- 18.Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014 Aug;3(3):123–128.
- 19.Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014 Aug;67(8):897–903.
- 20.Pereira MM, Andrade J. Epidemiological aspects of leptospirosis in a slum area in the city of Rio de Janeiro, Brazil. Search for leptospires and specific antibodies in rodents. Trans R Soc Trop Med Hyg. 1988 Sep;82(5):768–770. https://doi.org/10.1016/0035-9203(88)90231-3
» https://doi.org/10.1016/0035-9203(88)90231-3 - 21.Panti-May JA, Carvalho-Pereira TSA, Serrano S, Pedra GG, Taylor J, Pertile AC, et al. A Two-Year Ecological Study of Norway Rats (Rattus norvegicus) in a Brazilian Urban Slum. PLoS ONE. 2016;11(3):e0152511. https://doi.org/10.1371/journal.pone.0152511
» https://doi.org/10.1371/journal.pone.0152511 - 22.Santos NDJ, Sousa E, Reis MG, Ko AI, Costa F. Rat infestation associated with environmental deficiencies in an urban slum community with high risk of leptospirosis transmission Cad Saude Publica. 2017;33(2):e00132115.
- 23.Hamond C, Martins G, Lawson-Ferreira R, Medeiros MA, Lilenbaum W. The role of horses in the transmission of leptospirosis in an urban tropical area. Epidemiol Infect. 2013;141(1):33–35.
- 24.Lelu M, Muñoz-Zanzi C, Higgins B, Galloway R. Seroepidemiology of leptospirosis in dogs from rural and slum communities of Los Rios Region, Chile. BMC Vet Res. 2015;11:31.
- 25.Leon LL, Garcia RC, Diaz CO, Valdez RB, Carmona GCA, Velazquez BLG. Prevalence of leptospirosis in dairy cattle from small rural production units in Toluca Valley, State of Mexico. Ann N Y Acad Sci. 2008 Dec;1149:292–295. https://doi.org/10.1196/annals.1428.002
» https://doi.org/10.1196/annals.1428.002 - 26.Lall C, Kumar KV, Raj RV, Vedhagiri K, Vijayachari P. Prevalence and Diversity of Leptospires in Different Ecological Niches of Urban and Rural Areas of South Andaman Island. Microbes Environ. 2016;31(1):79–82. https://doi.org/10.1264/jsme2.ME15149
» https://doi.org/10.1264/jsme2.ME15149 - 27.Escandón-Vargas K, Osorio L, Astudillo-Hernández M. Seroprevalence and factors associated with Leptospira infection in an urban district of Cali, Colombia. Cad Saude Publica. 2017;33(5):e00039216.
- 28.Calderón A, Rodríguez V, Máttar S, Arrieta G. Leptospirosis in pigs, dogs, rodents, humans, and water in an area of the Colombian tropics. Trop Anim Health Prod. 2014;46(2):427–432.
- 29.Vanasco NB, Schmeling MF, Lottersberger J, Costa F, Ko AI, Tarabla HD. Clinical characteristics and risk factors of human leptospirosis in Argentina (1999–2005). Acta Trop. 2008;107(3):255–258.
- 30.Meny P, Menéndez C, Ashfield N, Quintero J, Rios C, Iglesias T, et al. Seroprevalence of leptospirosis in human groups at risk due to environmental, labor or social conditions. Rev Argent Microbiol. 2019;51(4):324–333. https://doi.org/10.1016/j.ram.2019.01.005
» https://doi.org/10.1016/j.ram.2019.01.005 - 31.Pelissari DM, Maia-Elkhoury ANS, Arsky M de LNS, Nunes ML. Revisão sistemática dos fatores associados à leptospirose no Brasil, 2000-2009. Epidemiol Serv Saude. 2011;20(4):565–574.
- 32.Barcellos C, Lammerhirt CB, Almeida MAB de, Santos E dos. Distribuiçäo espacial da leptospirose no Rio Grande do Sul, Brasil: recuperando a ecologia dos estudos ecológicos TT. Cad Saude Publica. 2003;19(5):1283–1292. https://doi.org/10.1590/S0102-311X2003000500007
» https://doi.org/10.1590/S0102-311X2003000500007 - 33.Carreño LA, Salas D, Beltrán KB. Prevalencia de Leptospirosis en Colombia: revisión sistemática de literatura. Rev Salud Publica. 2017;19(2):204–209.
- 34.Góngora A, Parra JL, Aponte LH, Gómez LA. Seroprevalencia de Leptospira spp. en Grupos de Población de Villavicencio, Colombia. Rev Salud Publica. 2008;10(2):269–278.
- 35.Nicolino RR, Lopes LB, Rodrigues RO, Teixeira JFB, Haddad JPA. Prevalence and spatial analysis of antileptospiral agglutinins in dairy cattle - Microregion of Sete Lagoas, Minas Gerais, 2009/2010. Arq Bras Med Vet Zootec. 2014;66(3):648–654.
- 36.Marteli AN, Genro LV, Diament D, Guasselli LA. Análise espacial da leptospirose no Brasil. Saude Debate. 2020;44(126):805–817.
- 37.Allan KJ, Biggs HM, Halliday JEB, Kazwala RR, Maro VP, Cleaveland S, et al. Epidemiology of Leptospirosis in Africa: A Systematic Review of a Neglected Zoonosis and a Paradigm for ‘One Health’ in Africa. PLoS Negl Trop Dis. 2015;9(9):e0003899. Available from: https://doi.org/10.1371/journal.pntd.0003899
» https://doi.org/10.1371/journal.pntd.0003899 - 38.Lau CL. Human Leptospirosis in Oceania. In: Loukas A. (ed). Neglected Tropical Diseases - Oceania. Neglected Tropical Diseases. Cham, Switzerland: Springer; 2016:177–192. https://doi.org/10.1007/978-3-319-43148-2_7
» https://doi.org/10.1007/978-3-319-43148-2_7 - 39.Machang’u RS, Mgode GF, Assenga J, Mhamphi G, Weetjens B, Cox C, et al. Serological and molecular characterization of leptospira serovar Kenya from captive African giant pouched rats (Cricetomys gambianus) from Morogoro Tanzania. FEMS Immunol Med Microbiol. 2004;41(2):117–121.
Publication Dates
- Publication in this collection
01 Sept 2023 - Date of issue
2023
History
- Received
18 Nov 2022 - Accepted
06 June 2023


 Source: Prepared by the authors based on the study data.
Source: Prepared by the authors based on the study data.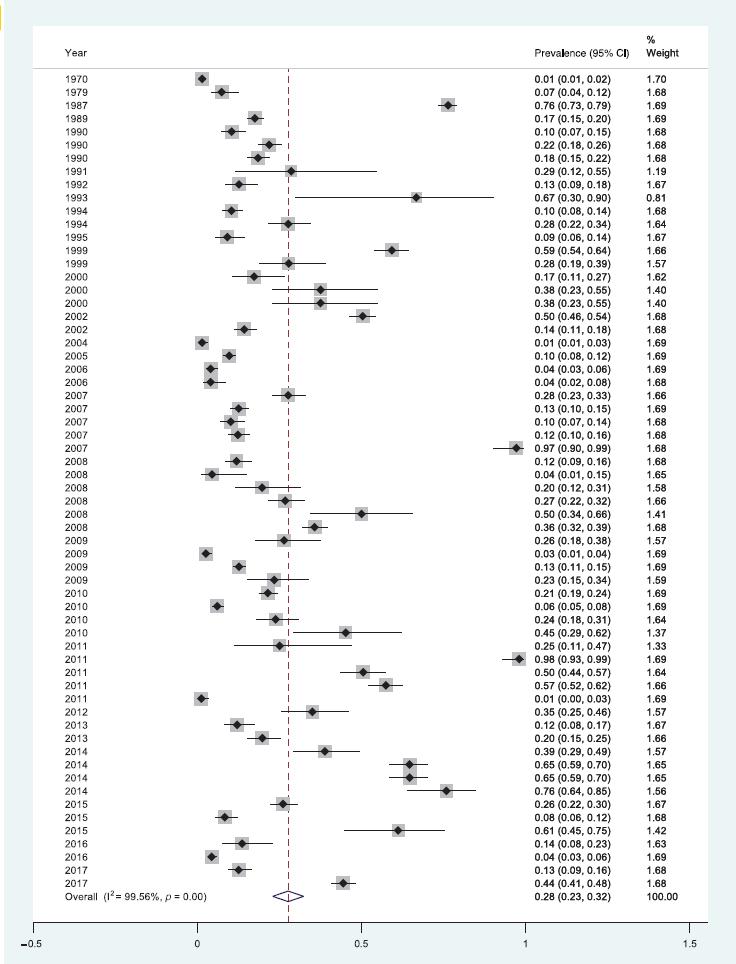 Source: Prepared by the authors based on the study data.
Source: Prepared by the authors based on the study data.